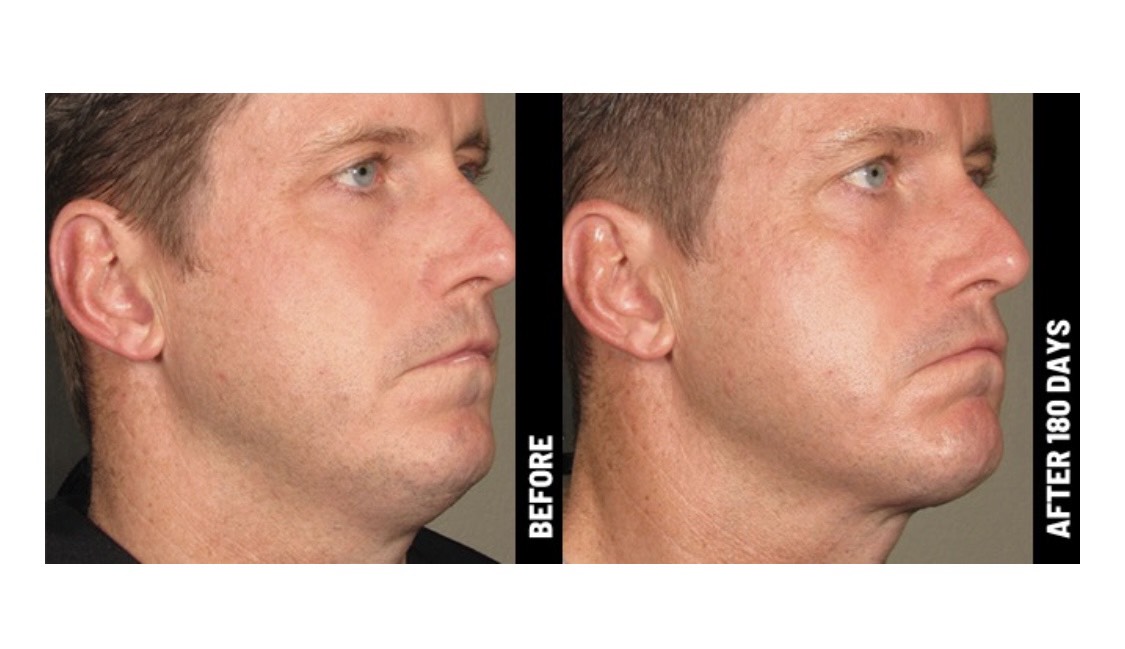
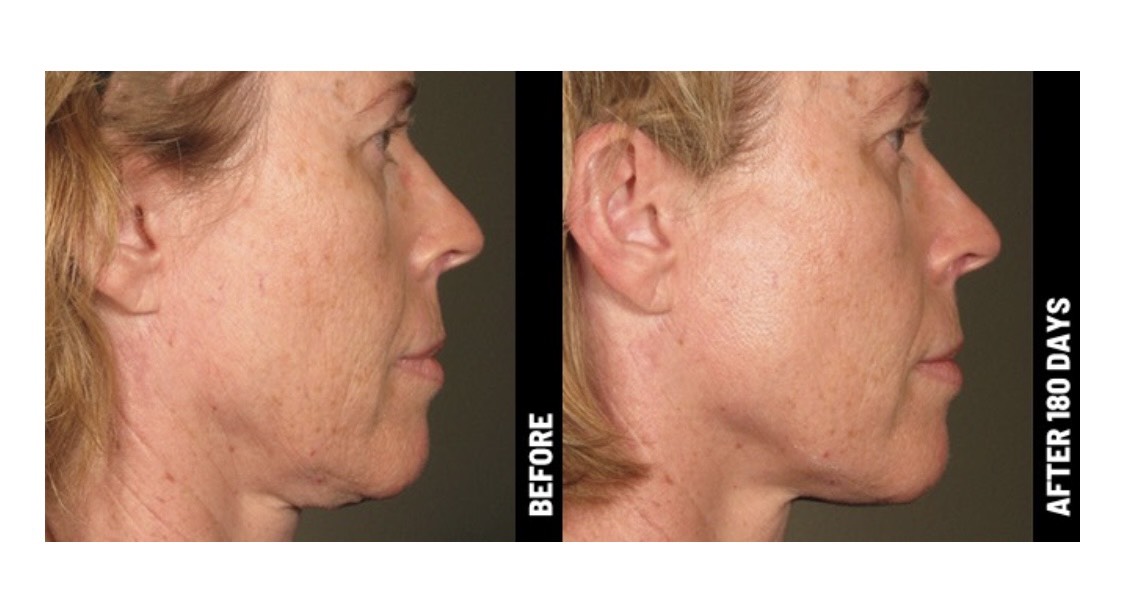
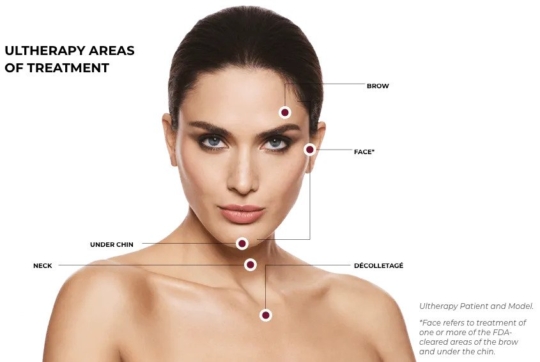
Ultherapy is FDA-cleared to lift the skin on the brow, neck, and under the chin, and improve lines and wrinkles on the décolletage.
Only Ultherapy is FDA-cleared to non-invasively lift skin on the neck, under the chin, and on the brow—and also improve lines and wrinkles on the décolletage! No wonder we’ve delivered more than a million treatments worldwide.

Ultherapy lifts as deep as surgical lift:-
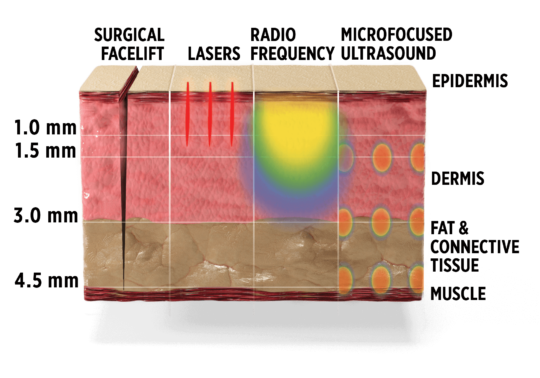 A DEEPER NONSURGICAL LIFT
A DEEPER NONSURGICAL LIFT
Ultherapy can precisely target at depths up to 4.5 mm, the same as surgery. This modality treats substantially deeper than other non-invasive procedures.
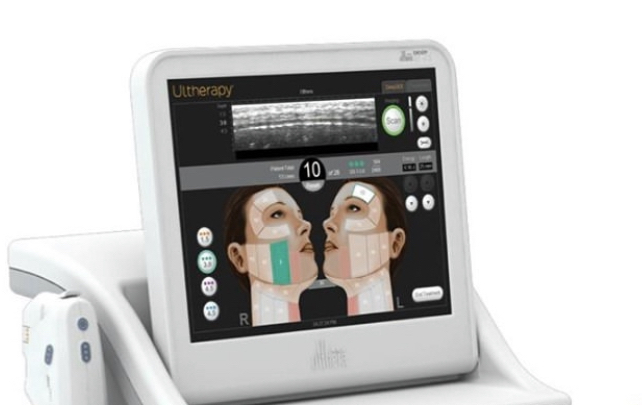
Ultherapy relies on ultrasound therapy to go deeper than other non-invasive treatments for collagen stimulation. And it leverages traditional ultrasound imaging so Ultherapy providers can see the layers of tissue they are treating. This ensures the treatment energy is delivered to where it benefits you most.
Ultherapy Stimulates Collagen Production:-
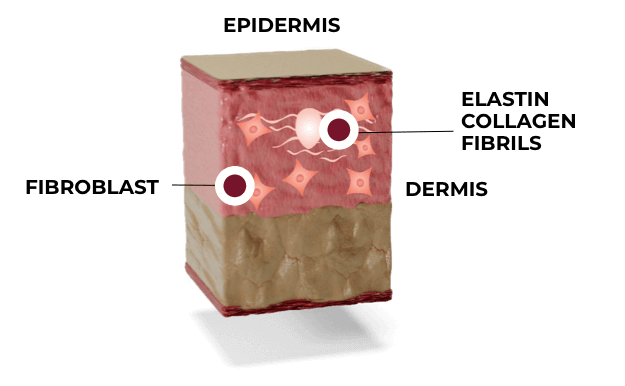
Ultherapy microfocused ultrasound triggers neocollagenesis—the body’s own healing response—initiating new collagen and elastin production.
Ultherapy increases collagen synthesis by 42%.
Data from a unique Ultherapy study, in which patients received Ultherapy treatment on only one side of the face, demonstrated an average 1.4 times increase in the rate of collagen production in the treated side vs. untreated. The rate of production for collagen Types I and III—those types most closely associated with the effects of aging—increased 42% in the side treated with Ultherapy.
Phases of neocollagenesis:

PHASE 1: INFLAMMATION
Activated upon Injury
Application of microfocused ultrasound energy creates TCPs, which denature collagen and initiate an inflammatory response. Macrophages engulf and digest injured tissue and release cytokines, which attract fibroblasts.
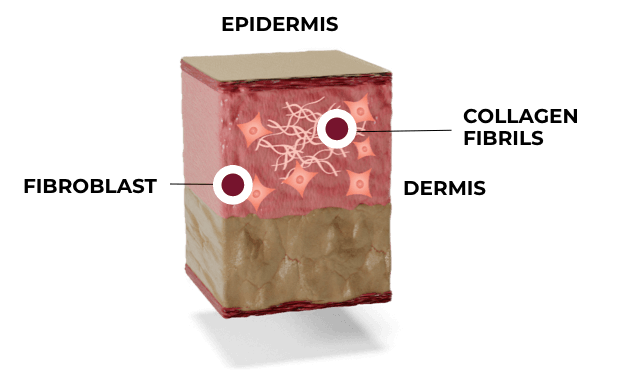
PHASE 2: PROLIFERATION
Typically starts within hours; can last for several weeks
Fibroblasts synthesize new collagen, mainly Type III, and other mediators important to rebuilding of the collagen matrix. Histological analysis of facial skin treated with Ultherapy shows a significant increase in collagen and elastin.
PHASE 3: MATURATION & REMODELING
Typically starts at 3 weeks post-injury; may last up to 1 year
Type III collagen is replaced by Type I, forming tight cross-links with itself and other proteins. This gradual collagen remodeling process is essential to the skin tightening and lifting provided by Ultherapy.
CONTRAINDICATIONS for Ultherapy
The Ulthera System is contraindicated for use in patients with:
• Open wounds or lesions in the treatment area
• Severe or cystic acne in the treatment area
• Active implants (e.g., pacemakers or defibrillators), or metallic implants in the treatment area
Treatment is not recommended directly over those areas with any of the following:
• Dermal fillers
• Thyroid gland, thyroid cartilage and trachea
• Major vessels
• Breast tissue or breast implants
The Ultherapy System has not been evaluated for use in the following patient populations, and therefore not recommended:
• Pregnant or breast feeding woman
• Children
• Those with the following disease states:
• A hemorrhagic disorder or hemostatic dysfunction
• An active systemic or local skin disease that may alter wound healing
• Herpes Simplex
• Autoimmune Disease
• Diabetes
• Epilepsy
• Bell’s Palsy
POTENTIAL SIDE EFFECTS of Ultherapy
Side effects reported in the clinical evaluation of the Ulthera System for the brow, submental (under the chin) area and neck treatment(s) were mild and transient in nature. These were limited to:
Erythema (redness): The treated area may exhibit erythema immediately following treatment. This typically resolves within a few hours of treatment.
Edema (swelling): The treated area may exhibit mild edema following treatment. This typically resolves within 3 to 72 hours of treatment.
Pain: Momentary discomfort may be experienced during the procedure while energy is being deposited. Post procedure discomfort typically resolves within 2 hours and 2 days. Tenderness to the touch is also possible and typically resolves within 2 days to 2 weeks of treatment.
Bruising: Mild bruising, which is caused by damage to soft tissue blood vessels, may occur occasionally and typically resolves within 2 days to 2 weeks of treatment.
Nerve Effects:
• Transient local muscle weakness may result after treatment due to inflammation of a motor nerve. This typically resolves in 2 to 6 weeks of treatment.
• Transient numbness may result after treatment due to inflammation of a sensory nerve. This typically resolves in 2 to 6 weeks of treatment.
• Transient pain, paresthesia and/or tingling may be experienced. This typically resolves in 2 to 6 weeks of treatment.
No permanent injuries to facial nerves have been reported during clinical trials.
Scarring: The possibility for scar formation (which may respond to medical care) may exist if incorrect treatment technique is used.
Side effects reported in the clinical evaluation of the Ulthera System for the décolleté treatment were mild and transient in nature.
These were limited to:
Erythema (redness): The treated area may exhibit erythema immediately following treatment. This typically resolves within a few hours of treatment.
Edema (swelling): The treated area may exhibit mild edema following treatment. This typically resolves within 3 to 48 hours of treatment.
Pain: Momentary discomfort may be experienced during the procedure while energy is being deposited. Post procedure discomfort typically resolves within 2 hours and 2 days. Tenderness to the touch is also possible and typically resolves within 2 days to 2 weeks of treatment.
Raised area of edema: The treated area may exhibit a localized area of linear visible edema following treatment. This typically resolves within 1 day to 3 weeks of treatment.
Bruising: Mild bruising, which is caused by damage to soft tissue blood vessels, may occur occasionally and typically resolves within 3 days and 3 weeks of treatment.
• Transient Sensory Nerve Effects (as a result of inflammation of the nerve):
• Paresthesia and/or numbness may be experienced and typically resolves within 4 days to 5 weeks of treatment.
• Tingling may result after treatment and typically resolves within 3 to 5 days of treatment
• Itching may result after treatment and typically resolves within 1 to 3 weeks of treatment.
No permanent injuries to facial nerves have been reported during clinical trials.
Scarring: The possibility for scar formation (which may respond to medical care) may exist if incorrect treatment technique is used.
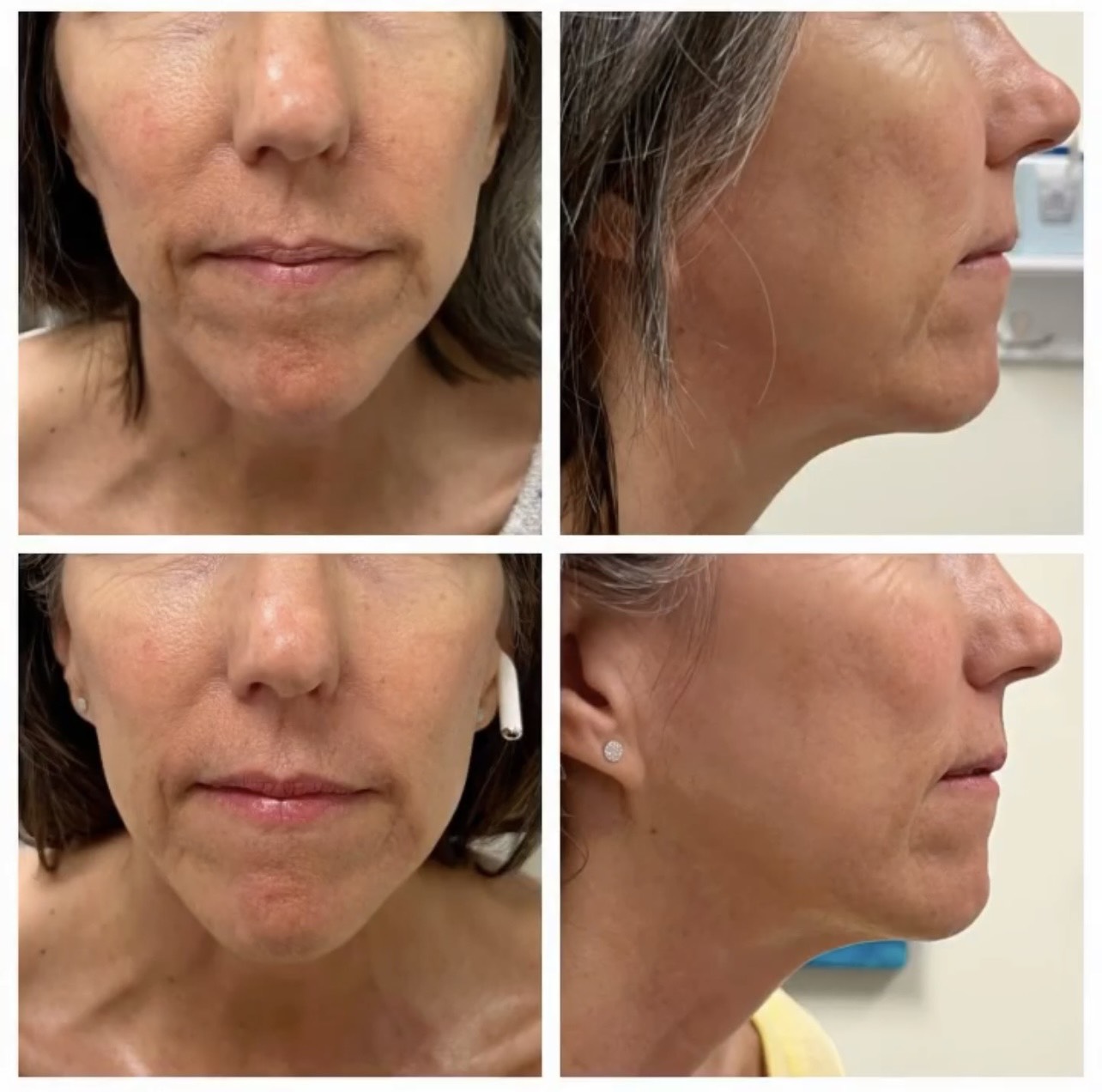
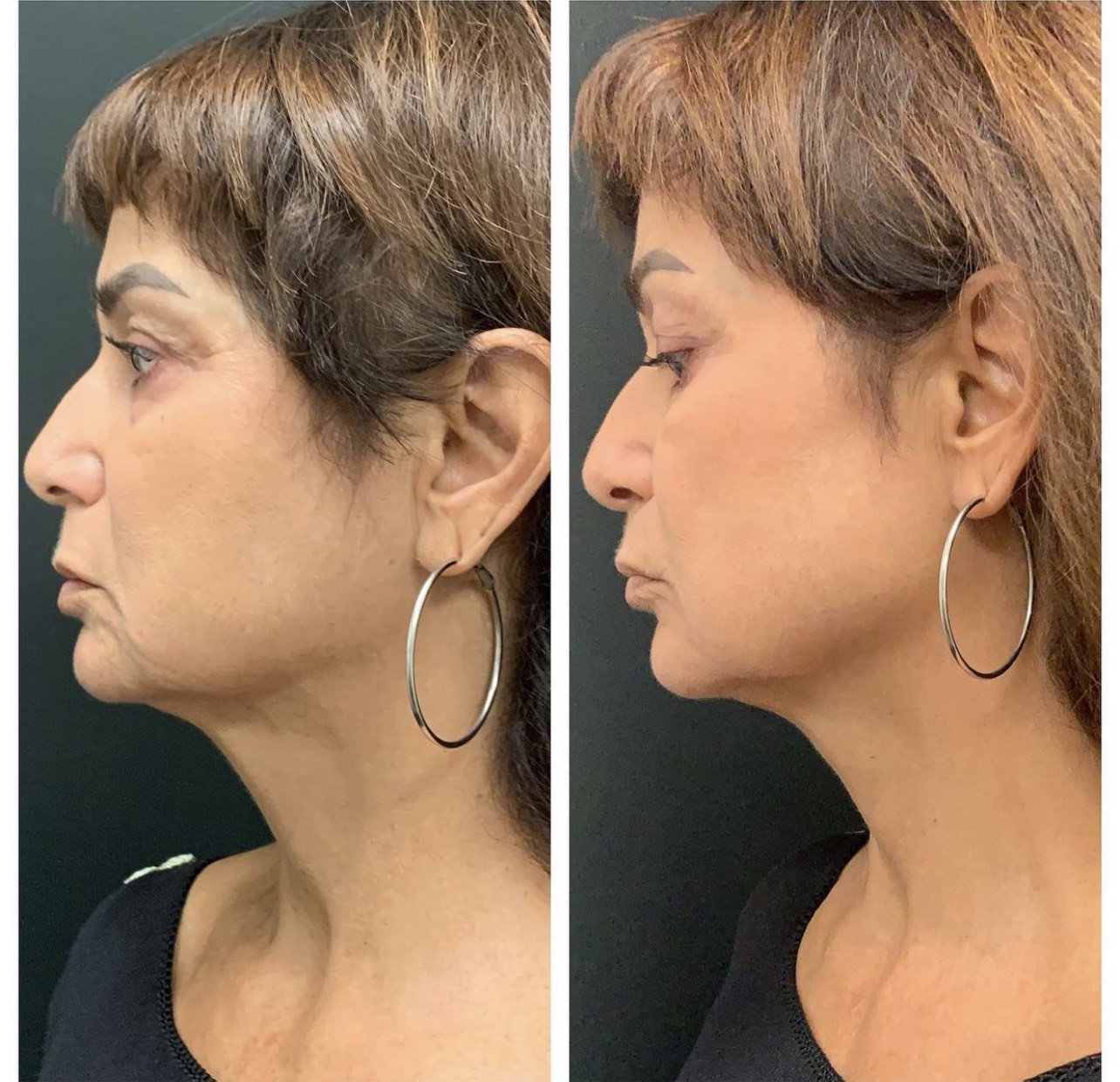
Ultherapy treatment of the face and neck improved the under eye, jowl, chin and neck sagging.
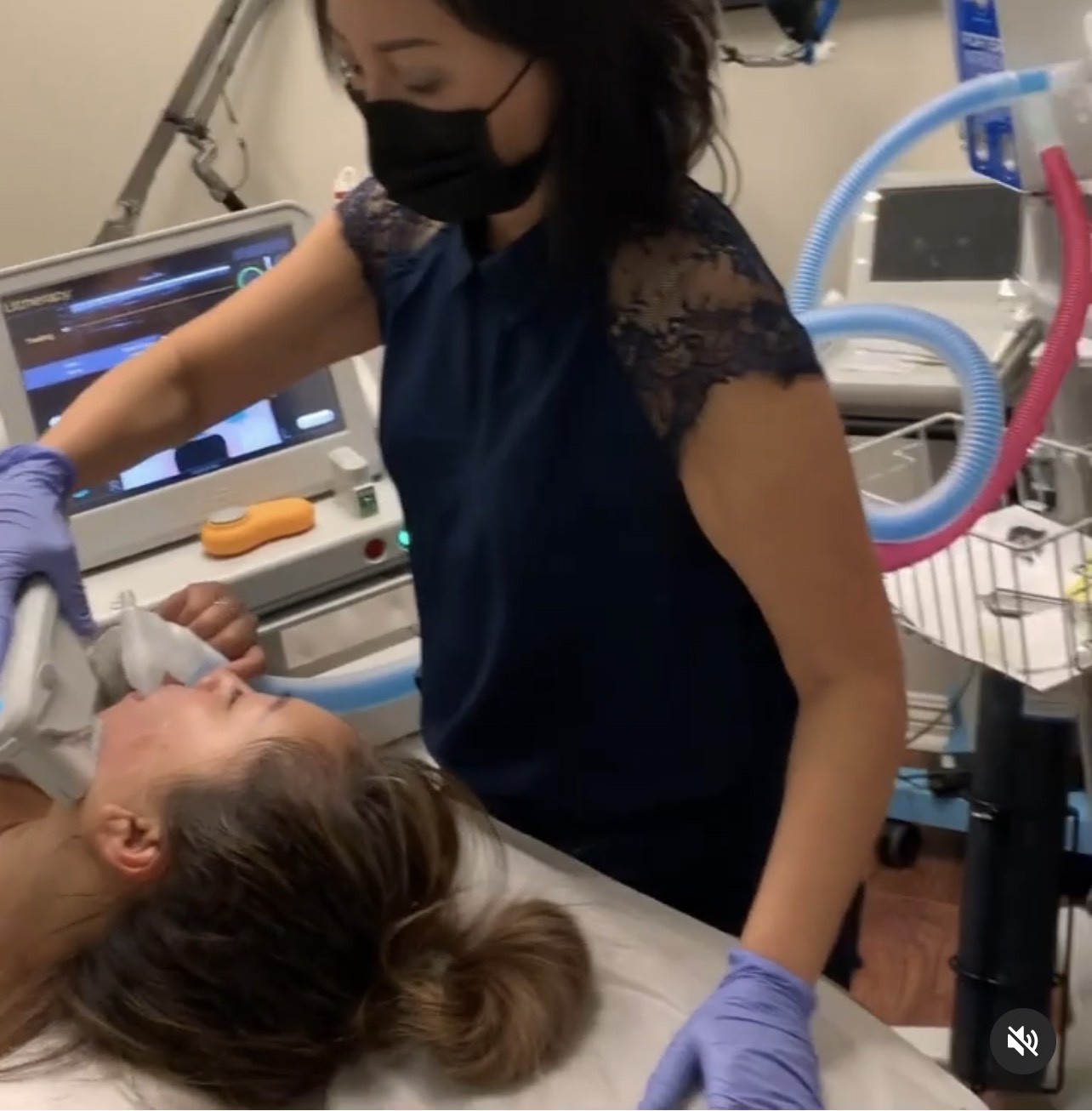
Client experience for Ultherapy treatment has much improved since we incorporated Nitrous Oxide relaxation system at Bellatudo Skin and Wellness Center. Nitrous Oxide system is client controlled, demand-based, activated only when the client breathes in through the disposable mouth piece and the tubing. We are loving it!