What is KYBELLA®?
KYBELLA® is a prescription medicine used in adults to improve the appearance and profile of moderate to severe fat below the chin (submental fat), also called “double chin.”
KYBELLA injection is a cytolytic drug, which when injected into tissue physically destroys the fat cell membrane causing fat cell lysis (breaking down).
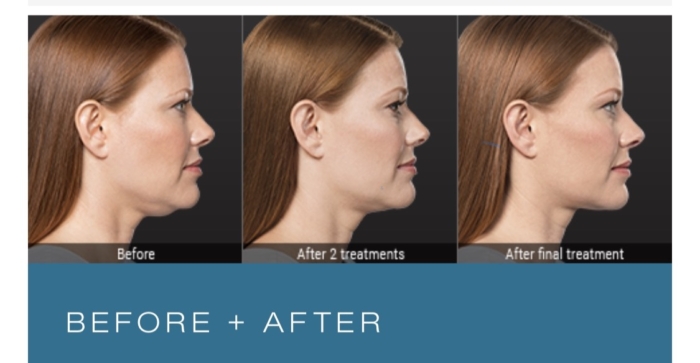
The effects of KYBELLA is permanent, and the reduction of under chin fat pad is permanent as long as the person does not add new fat cells by gaining weight.
The most common side effects of KYBELLA include:
• swelling • redness
• pain • areas of hardness in the treatment area • numbness
The rare serious side effects of KYBELLA include:
. Marginal mandibular nerve injury manifested as an asymmetric smile or facial muscle weakness (paresis), which resolves spontaneously mostly within 8 weeks
. Difficulty swallowing (dysphagia) in the setting of administration site reactions, e.g., pain, swelling, and induration of the submental area, which resolves mostly within a week
. Injection site hematoma/bruising
. Injection site ulceration and necrosis when injection is done too superficially
KYBELLA injection is injected into subcutaneous fat tissue in the submental area using an area-adjusted dose of 2 mg/cm .
• A single treatment consists of up to a maximum of 50 injections, 0.2 mL each (up to a total of 10 mL), spaced 1 cm apart. Each vial of KYBELLA is 2 ml, and the maximum a single session can deliver is 5 vials. Usually 1-2 vials at a session is good to avoid too much swelling.
• Depending on the size of the under chin fat pad, 1-6 single treatments may be administered at intervals no less than 1 month apart.
Metabolism and Excretion
Deoxycholic acid is naturally made by our body as a product of cholesterol metabolism and is excreted intact in feces. Deoxycholic acid from KYBELLA joins the natrally occuring Deoxycholic acid and is excreted together.
A greater proportion of KYBELLA-treated subjects had at least a 10% reduction in submental (under chin area) fat volume as compared to placebo-treated subjects , 43% vs 5%, respectively.
The overall patient-reported satisfaction and self-perceived visual attributes showed greater improvement in the KYBELLA group than in the placebo group.
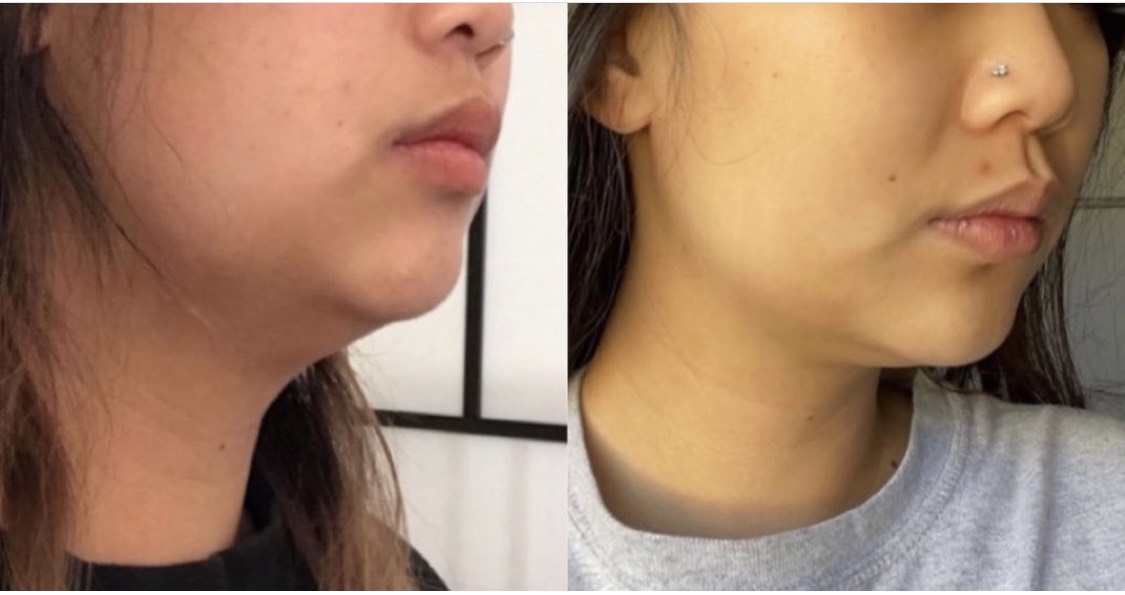
Kybella injection for chin fat pad reduction to improve the chin and jawline profile, after 2 vials
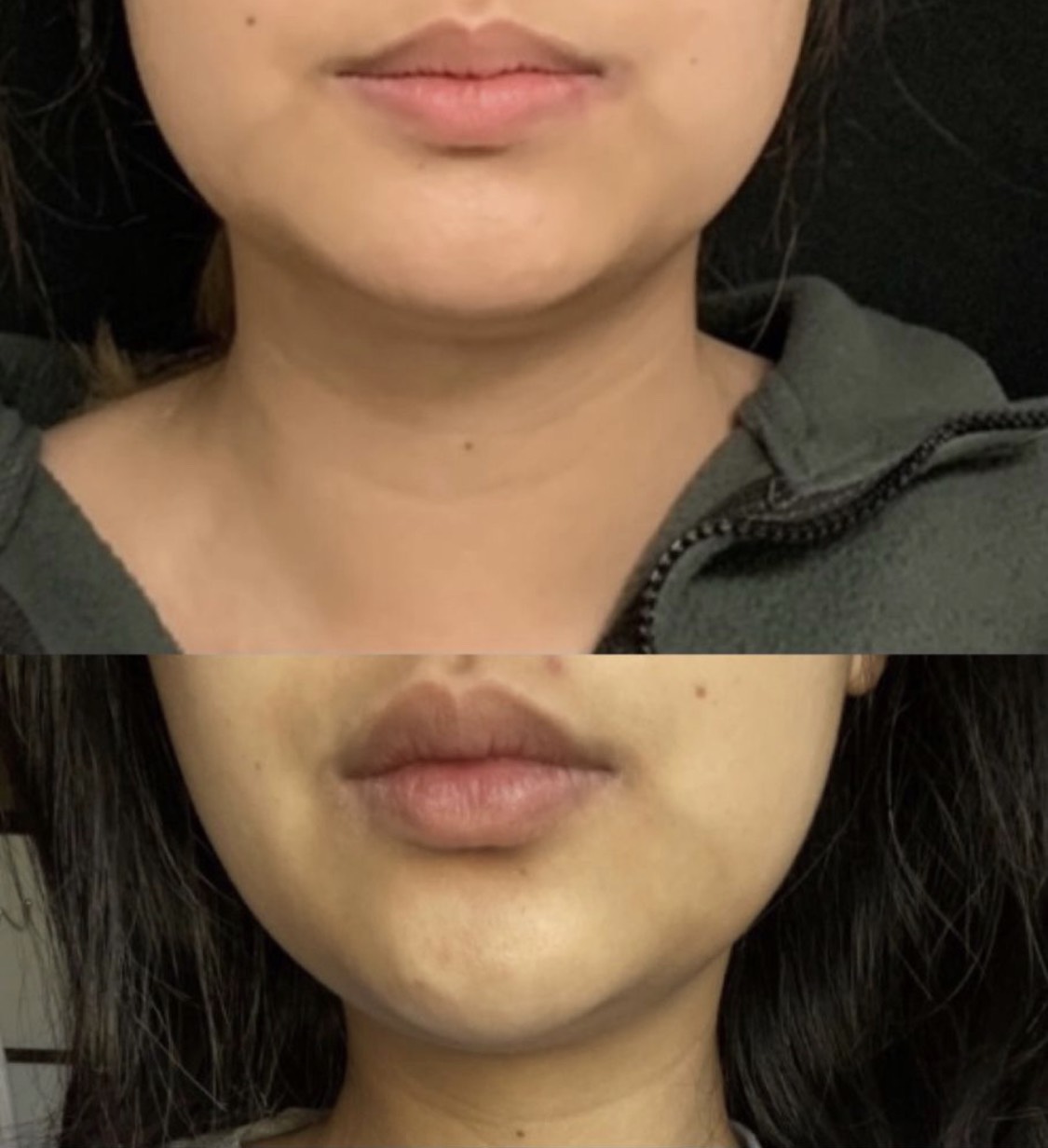
Kybella injection for chin fat pad reduction to improve the chin and jawline profile, after 2 vials